Accumold is pleased to announce that it will be presenting an MD+DI / Qmed+ Webinar focused on micro manufacturing for wearable diabetes devices. The webinar — discussing a thin-wall and overmolding case study — will be held on Thursday, June 27, 2024 14.00 Eastern Daylight Time (20.00 CET), and will be hosted by Paul Runyan, VP Sales and Marketing and AJ Pruitt, Technology Manager at Accumold. Interested parties can register HERE.
Small and thin-walled cannulas play a pivotal role in wearable diabetes devices due to their significance in minimizing patient discomfort and optimizing medical procedures. The interactive online event will show engineers, designers, project managers, and procurement teams how injection molding can offer better solutions and reduced pricing.
Paul Runyan says, “After 5 years of painstaking work, Accumold has developed a way to micro injection mold thin wall cannulas in very high volumes. Previous to this innovative approach to production, cannulas were typically produced via an extrusion process, which is at the same time expensive, prone to high fall out rates, and which does not lend itself to the high volumes required by medical device OEMs for some applications. What we found during 5 years of learning was that to successfully injection mold thin-walled cannulas, while material choice is critical, so too is the cannula design and aspect ratios which we will discuss in detail during the MD+DI / Qmed+ webinar.”
Accumold discovered that to successfully micro mold thin wall cannulas, several critical design for manufacturability (DFM) considerations needed to be addressed. Ensuring uniform wall thickness was paramount, as variations could lead to warping, cooling inconsistencies, and inadequate filling. Proper gate placement was also essential, influencing material flow and minimizing stress points, while suitable venting channels were crucial to prevent air traps that could result in surface defects. Incorporating appropriate draft angles facilitated seamless ejection from the mold and prevented potential damage.
The company also found that an appropriate aspect ratio directly impacted the manufacturability and quality of the molded part. Maintaining a balanced aspect ratio was essential to avoid challenges associated with flow dynamics, cooling, and structural integrity. An excessively high aspect ratio could lead to difficulties in material flow and cavity filling, potentially resulting in uneven thickness and defects. Conversely, an aspect ratio that was too low might hinder proper cooling and cause warping, making it vital to strike the right balance that promoted both accurate molding and structural stability.
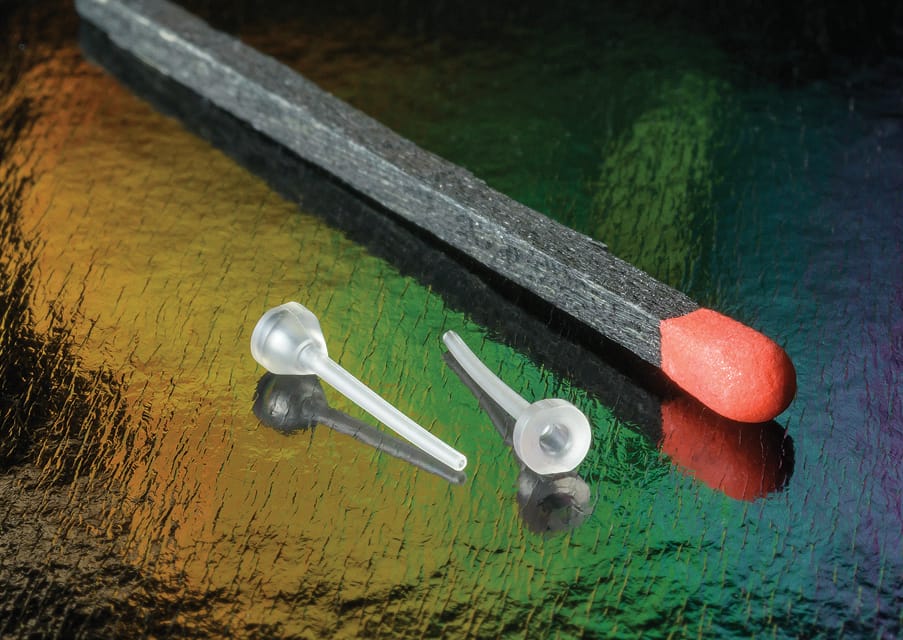
AJ Pruitt says, “Beyond the choice of materials, design considerations, and aspect ratios, there was one other variable that has been critical to Accumold’s success in the high-volume manufacture of thin-walled cannulas, and that the use of its proprietary micro molding presses, developed over generations. Cannulas were molded on conventional presses and on Accumold’s presses, and it was discovered that conventional micro molding presses had problems with non-fill and flash. Through the use of our fully automated in-house developed micro molding presses and 16 cavity micro mold tooling, we achieved reliable, repeatable, and high volume production of 40 million parts a year from a single production cell.”
Using Accumold’s revolutionary and proprietary micro injection molding process, cannulas can now be produced at high volume with simplified all-plastic bodies, and heads designed to maximize manufacturing efficiencies and product integration. Register for the MD+DI / Qmed+ webinar now to learn more and to see how micro molded thin-wall cannulas can stimulate innovation in the design of your wearable diabetes devices.