Due to go live 26 May 2020, many medical device moulders, particularly SMEs with limited in-house resources, are still getting to grips with the impact of the EU Medical Device Regulations (MDR). Designed to make products more easily traceable by patients and the care supply chain, many perceive the labelling upgrade investment to be a challenging and costly undertaking. Appling to all medical device products sold in the EU, even if made outside of the EU, failure to comply could result in products removed from sale after the EU MDR deadline.
With limited time left, Nigel Flowers, Managing Director at Sumitomo (SHI) Demag UK, explains to moulders that they may have the data driven technology in place to ensure compliance but are unsure if it has been activated. He answers some common questions to help put minds at rest.
Q: What is needed to achieve traceability?
A: With EU MDR, every Class III (e.g. implants and pacemakers) and Class IIa/b devices (e.g. surgical clamps and trachotomy tubes) or its packaging must be issued with a Unique Device Identification (UDI). Given that healthcare is a high liability market, it requires a fingerprint style approach to traceability. A UDI label must be directly attached to a medical device or to its packaging. Additionally, labels in the future will need to include twoidentifiers: a device identifier (DI) that identifies the labeller and the specific version or model of a device, plus a production identifier. This variable portion of the UDI needs to include the given lot or batch number, serial number, date of manufacture, expiry date, etc.
Q: What technology does a moulder need?
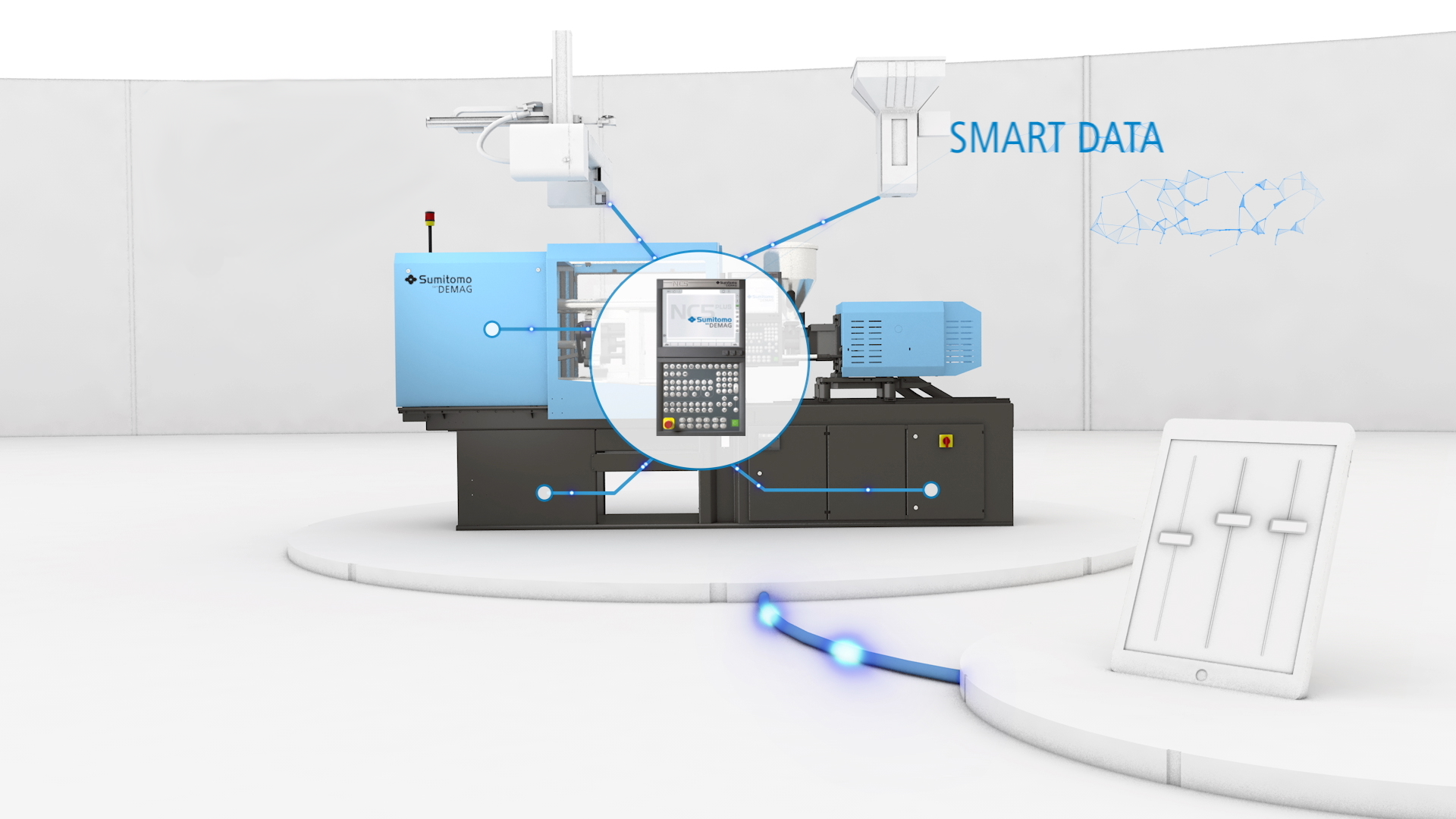
A: In Mould Decorating is an automated way toissue mass-produced medical devices with globally-compliant UDIs. It is like issuing each moulded medical device component with its unique birth certificate, with all processing data held securely by a manufacturing executive system (MES). It means that any potential quality defect, which might not be picked up for several months, or even years, can be tracked back to the very day and cycle it was manufactured to achieve item-level traceability and conduct root cause analyses on parts and components. Connectivity to a Management Executive System (MES) is vital.
Q: Is real-time traceability important?
A: Yes, having an auditable supply chain traceability system is not purely about compliance and providing mandatory information. Due to globalised production platforms, it’s becoming increasingly imperative to limit operational risk exposures with targeted rather than mass recalls. For high value components or when the margin for error is zero and patient safety could be at risk, real-time traceability provides the means to limit recall exposure by improving end-to-end process transparency. Real-time traceability also enables you to call up data and verify the exact settings used on the exact injection moulding machine, when that individual plastic part was made. This can enhance process monitoring and enables moulders to keep a closer watch on risk management, mitigation and containment.
Q: Do I need to make modifications to my existing machinery?
A: It will depend upon the age of your machinery, but the latest generations of injection moulding machines are often quite advanced with smart data capture integrated. UK customers will also be able to benefit soon from the digital MyConnect software, which can monitor machine availability, productivity, traceability and processing decisions taken. A Launchpad for data-driven efficiency improvements, as well as troubleshooting tools, traceability features like myLifeCycleLog stores a full archive of past service requests and actions taken.
Q: Are there specific user parameters I need to implement?
A: The key areas that might impact a stable process include changes in pressure, temperature, flow rate and cooling rates. To ensure these processes are not compromised, the latest generation medical packages we offer limit the processing ranges that operatives can adjust. For example, the IntElect S 180-tonne machine unveiled at K 2019 adheres to the explicit ISO 13485 medical device quality management and validation standards and introduces new user parameters. This helps to ensure that processes are kept within specified ranges and operators cannot make adjustments unless they have been granted authorisation.
Q: How does automation facilitate traceability?
A: Using robotics is central to the in-line manufacturing process, as even a single instance of applying an incorrect code can be a major liability. Modern medical cells typically integrate all of the elements needed in a turnkey cleanroom cell, including plastic injection processing, IMD, robotics and data capture and management. The automation solution deployed will vary depending on the moulding application. For larger medical components it might be a 6-axis robot, which holds the part while codes are etched or bonded onto the component. These are generated by the MES holding system, which reconciles the machine processing data and generates a code or data matrix. In addition to the exact production date and time, process data that’s recorded includes the injection and dosing time, melt cushion, injection pressure and temperature.
For smaller micro-medical parts, such as pipettes, a side entry robot may be used. At K 2019, Sumitomo (SHI) Demag’s medical showcase featured a complete turnkey medical moulding cell with a Hekuma side entry robot with advanced batch tracking and contact-free camera inspection.
Programmed to demould and rapidly place components individually in their corresponding cavity assigned racks, the Hekutipprocess ensures that if an issue with a specific cavity arises, the rack containing all corresponding cavity parts can be isolated and the rack recalled. This gripper system concept is capable of removing 64 pipettes in less than 0.6 seconds. After each rack is filled with 96 pipettes, a camera visually inspects the components from multiple angles ensuring there are no holes or burrs present.