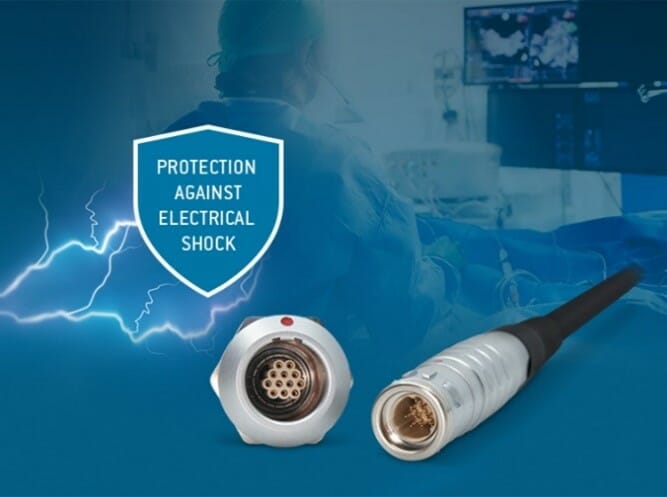
Medical equipment manufacturers are increasingly turning to ODU, to provide specific solutions to their ever-tightening technical requirements.
For instance, the ODU MINI-SNAP® portfolio now includes inserts that are specifically designed to comply with the levels of user and patient protection prescribed in the IEC Standard IEC 60601-1 (2 MOOP / 2 MOPP) – making them the first choice for anyone looking for a circular connector with a metal housing for medical applications.
Approval procedures to comply with IEC 60601-1 for electrical medical systems are highly complex.
So, involving ODU in the design-in process from the outset can both lower development costs and significantly simplify the risk management process.
Furthermore, approval procedures can be fast-tracked when components are already fully compliant with IEC 60601-1 upon delivery – another benefit of this connector.
Features:
- Robust metal housing
- Advanced electromagnetic shielding capabilities
- High mechanical strength
- Available in 3 sizes with a wide range of configuration options
- Reverse-gender use possible
- Rated at 7A

Other IEC 60601-1-compliant products include ODU MEDI-SNAP® and ODU MINI-MED®.
Also, a white paper on IEC60601-1 is available to download.
To further ensure a completely hygienic and resilient medical system, ODO also offer silicone over-moulded solutions – a complete system of connectors, cable assembly, over-moulding and optional labelling. The smooth surface prevents any sticking or the stick-slip effect, guarantees easy cleaning and meets absolute hygiene standards.
The system can withstand up to 500 autoclave cycles and is subject to medical technology testing according to ISO 10993-5. In addition, the smoothly transitioning over-moulding ensures bend protection.
Learn more View video now Download brochure
So, if you’re facing a connector or packaging conundrum, then why not drop by Stand K8 and tell them about your problems – they may have an answer.