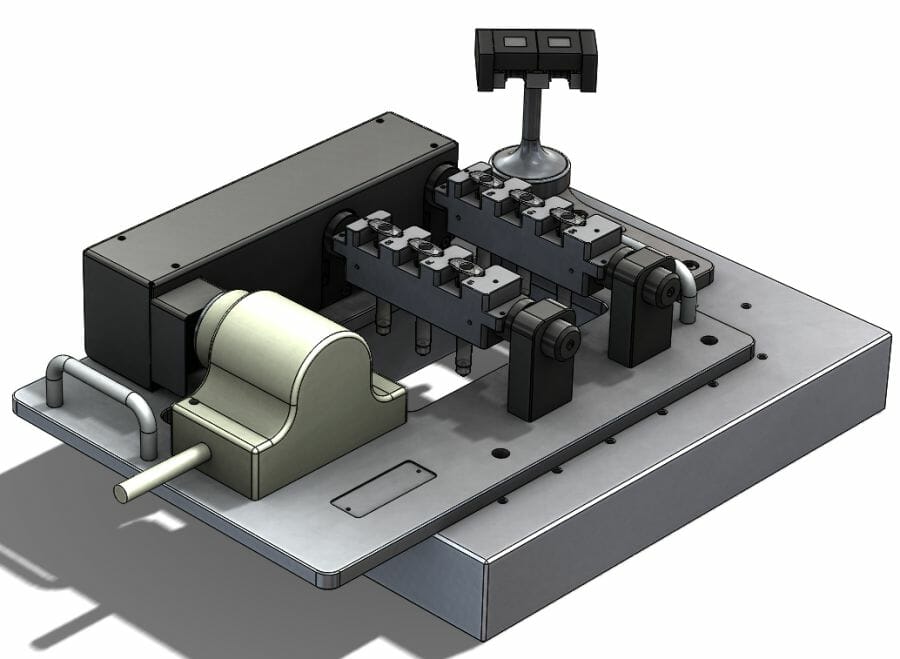
According to the McKinsey Center for Government, a medical device manufacturer experiences an average ten per cent drop in share price after a single, major product recall event. Remaining compliant is key to avoiding recalls, so providing ridged support during medical device quality testing stops unwanted movement and ensures testing is compliant. Here Mike G John CEng IMechE, head of engineering at industrial metrology supplier The Sempre Group explains the importance of fixtures in the medical device industry.
Medical device manufacturers understand the importance of a reliable manufacturing process, which is fully compliant with industry regulations, such as the biocompatibility regulation ISO 10993. Naturally, this compliance must include the fixtures that secure the components during quality testing to ensure ISO/IEC 17025, which requires competent testing and calibration, throughout the whole manufacturing process.
When measuring small, intricate components, Original Equipment Manufacturers (OEMs) get the most accurate results quickly when the part under inspection is secured. Fixtures, for example, are key when inspecting medical components, such as syringes, because they provide 360-degree access and keep them in place without exerting too much pressure and damaging the part. This is particularly important when using a touch probe, like the Micro-Vu with touch probe accessory, as it requires access to all areas of the component for visual inspection and internal tactile probing. These techniques can guarantee the measurements of components conform to the same standard as when it was qualified.
Choosing a fixture
OEMs have two choices of fixture, static or automated. Manual fixtures require operators to physically turn the fixture between each measurement, which can be a very time-intensive process. Both techniques have their place, but automated fixturing can deliver considerable benefits where it is suitable.
Automated fixtures take out the time-consuming process of manually turning the spar between measurements. The automated fixture integrates a high precision rotary indexer that automatically turns the parts. The operator does not have to wait for the measurements to take place, so can carry out jobs that require human intervention, such as data analysis.
Bespoke fixturing solutions
Manufacturers will not always be able to find an off-the-shelf solution that suits their application. If the part has a complex geometry, like a hip joint, it can become unstable in a standard modular fixturing system, which will alter the accuracy of the measurement.
In these circumstances OEMs can work with a fixturing provider to create a bespoke solution that securely holds the part and is compliant with medical regulations, reducing the risk of human error and improving accuracy. Sourcing external expertise provides manufacturers with the R&D, testing and production skills that avoid the risk and time-consuming process of designing the fixtures in-house.
Metrology providers can also integrate automated SPC software, such Prolink, with the fixturing process to automatically measure the part and store the data in a single, centralised data collection point. Engineers can review real-time data at any point in the production process and analyse it to prove compliance and accuracy. The software also automatically produces first article inspection (FAI) reports at the click of a button that manufacturers can share with stakeholders.
Contacting a metrology one stop shop, such as The Sempre Group, allows OEMs to have an end-to-end turnkey solution to their metrology process. Metrology providers can assess quality testing needs and make recommendations, create the required fixtures, validate the parts based off industry regulations and deliver, calibrate and install the technology and software.
To find out more about integrating metrology into the medical device manufacturing process, read our latest white paper www.TheSempreGroup.com/metrology-in-medical-whitepaper